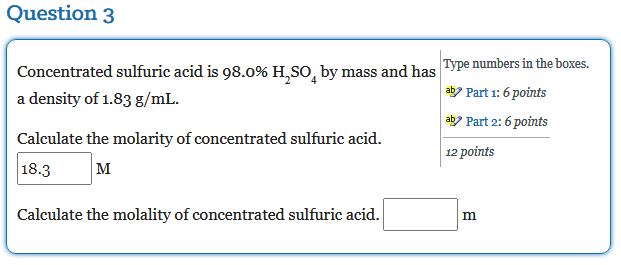
SOLVED:The concentrated sulfuric acid we use in the laboratory is 98.0 percent H2 SO4 by mass. Calculate the molality and molarity of the acid solution. The density of the solution is 1.83

a bottle of concentrated sulphuric acid (density of 1.80 g cm-3) is labelled as 86% as weight. What is - Brainly.in

Concentrated sulphuric acid has density of 1.9 g/mL and 99% H2SO4 by mass. Calculate the molarity of the acid.
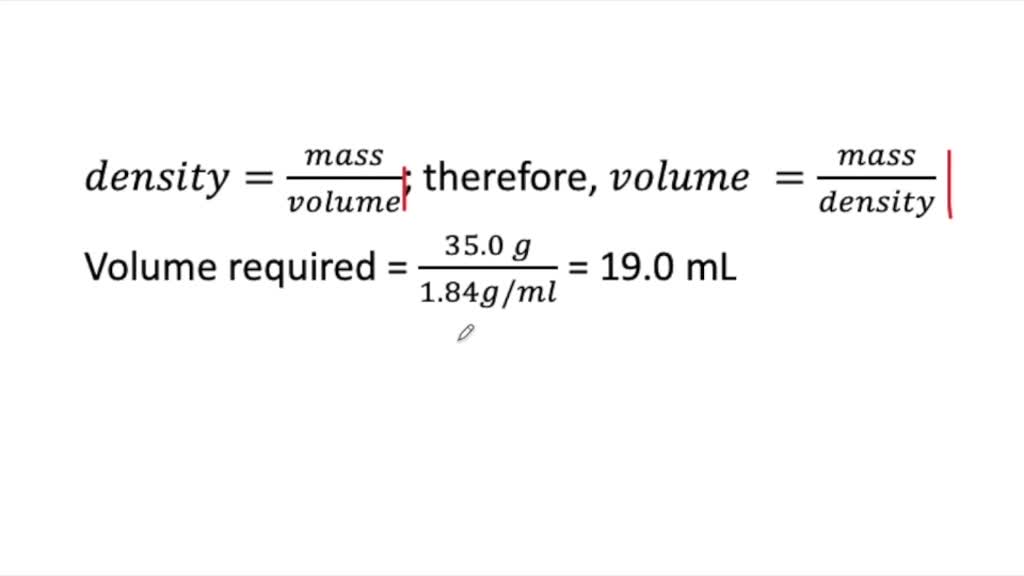
SOLVED:A chemist needs 35.0 g of concentrated sulfuric acid for an experiment. The density of concentrated sulfuric acid at room temperature is 1.84 g / mL. What volume of the acid is required?
What is the volume of concentrated H2SO4 of specific gravity 1.84 and containing 98% H2SO4 by weights that would contain 40 gm of pure H2SO4? - Quora
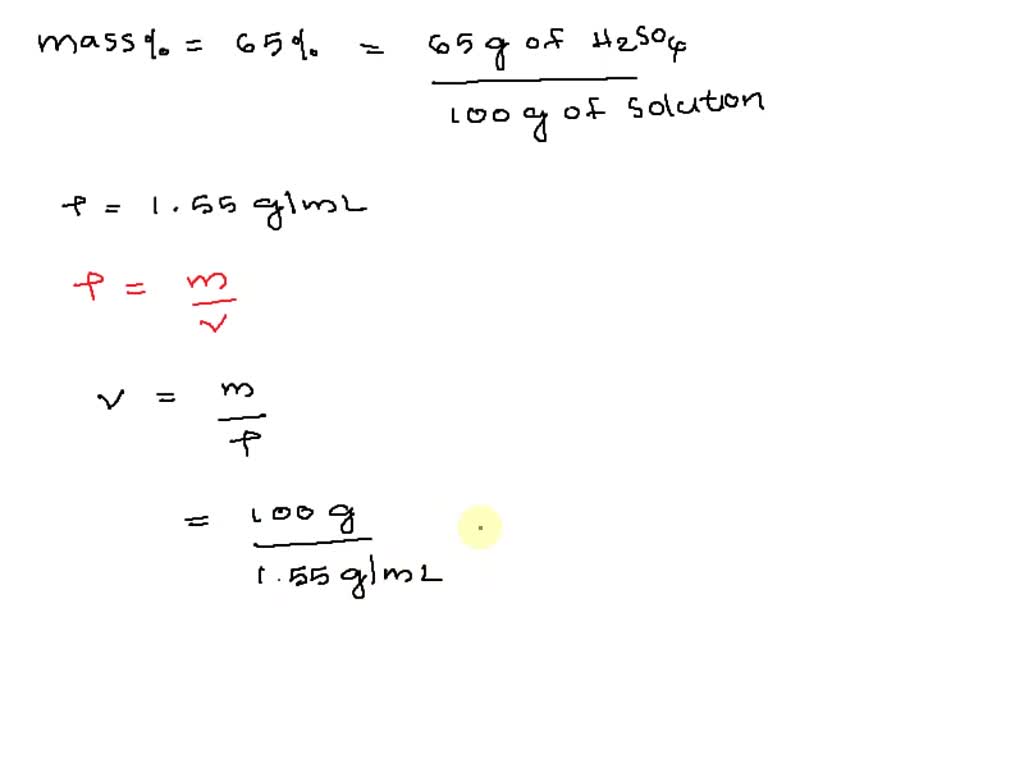
SOLVED: A concentrated sulfuric acid solution is 65.0% H2SO4 by mass and has a density of 1.55 g/mL at 20°C. What is the mass of 9.00 L of the concentrated sulfuric acid

What is the molarity of concentrated sulfuric acid if it is 96% by mass H2so4 and has a density of 1.84g/mL? - Quora

Concentrated aqueous sulphuric acid is 98% H2SO4 by mass and has a density of 1.80 gm L^-1 . What is the volume of acid required to make one litre of 0.1 M H2SO4 solution?

Laboratory grade concentrated sulphuric acid has a density 1.82g cc^(-1) .Weight percentage of acid is 98. Calculate the normality of solution.