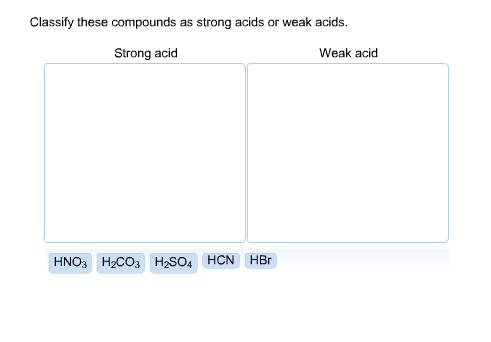
HCN is a weak acid ( Ka = 6.2 × 10^-10 ) ,NH4OH is a weak base ( Kb = 1.8 × 10^-5 ) . A 1.00 M solution of NH4CN would be:
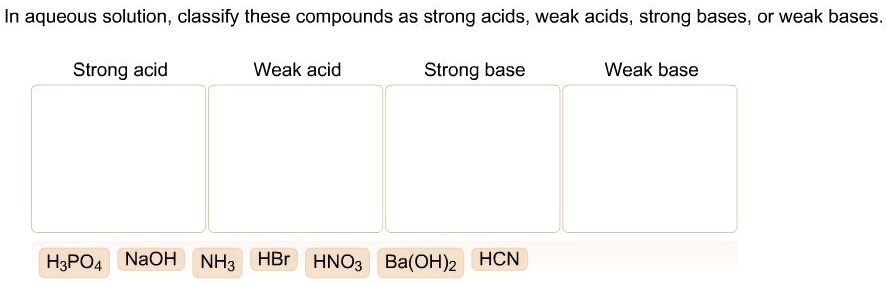
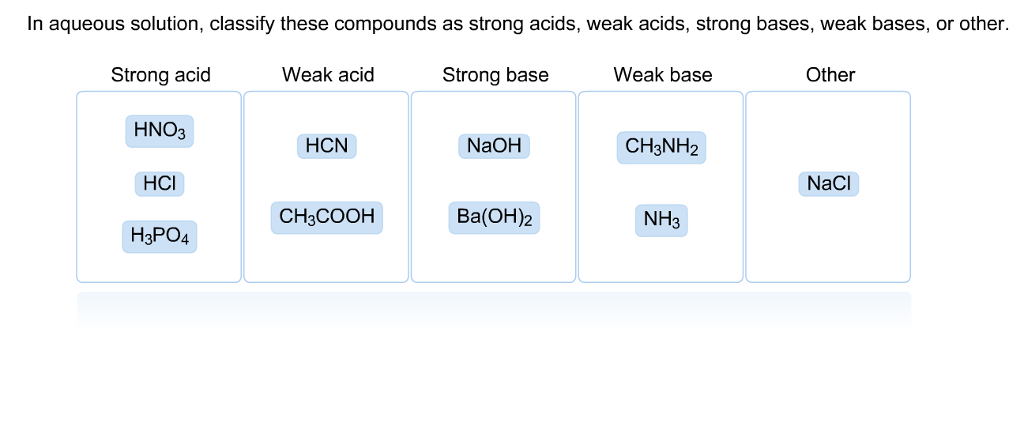
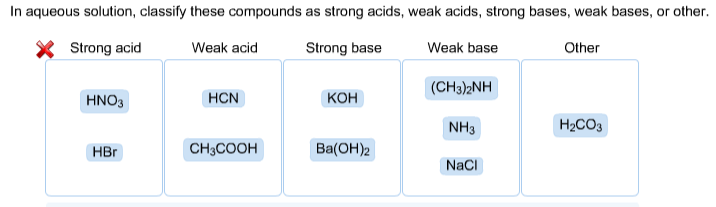
SOLVED: In aqueous solution, classify these compounds as strong acids, weak acids, strong bases or weak bases Strong acid Weak acid Strong base Weak base HaPO4 NaOH NH3 HBr HNO3 Ba(OH)z HCN

HCN is a weak acid ( Ka = 6.2 × 10^-10 ) ,NH4OH is a weak base ( Kb = 1.8 × 10^-5 ) . A 1.00 M solution of NH4CN would be:
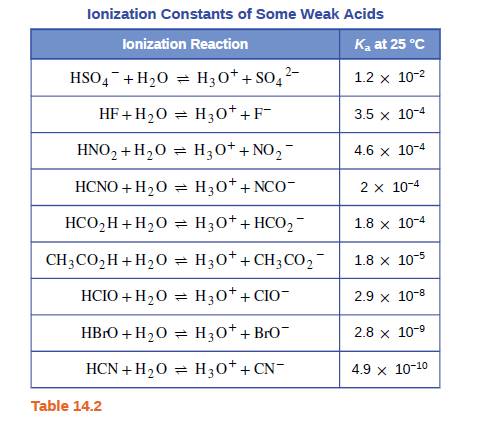
Both HF and HCN ionize in water to a limited extent. Which of the conjugate bases. F“ or CN”, is the stronger base? See Table 14.2. | bartleby

Identify the stronger acid in each pair: Part A: NH^+4 or H3O^+ Part B: H2SO4 or HCN Part C: H2O or H2CO3 | Homework.Study.com
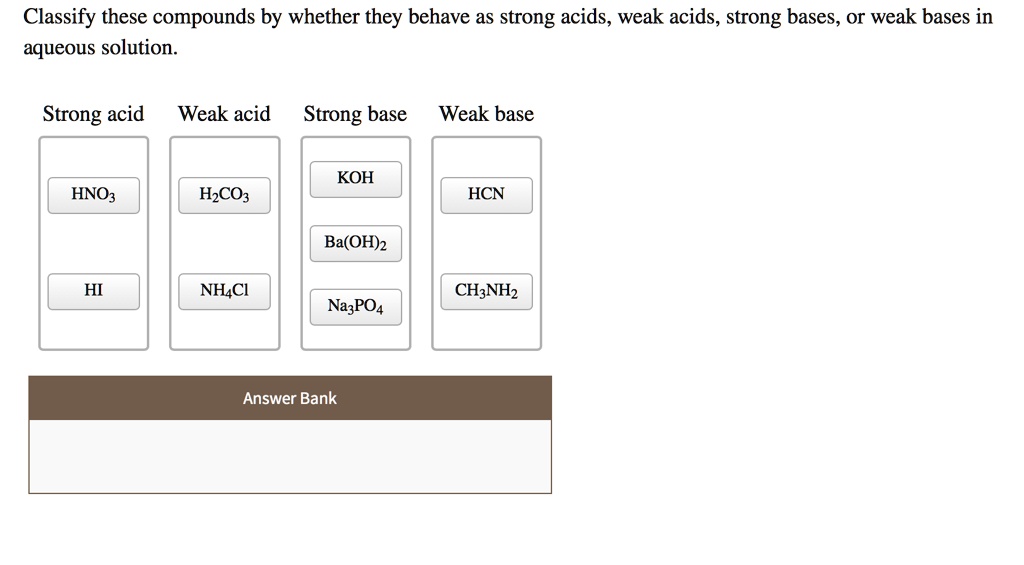
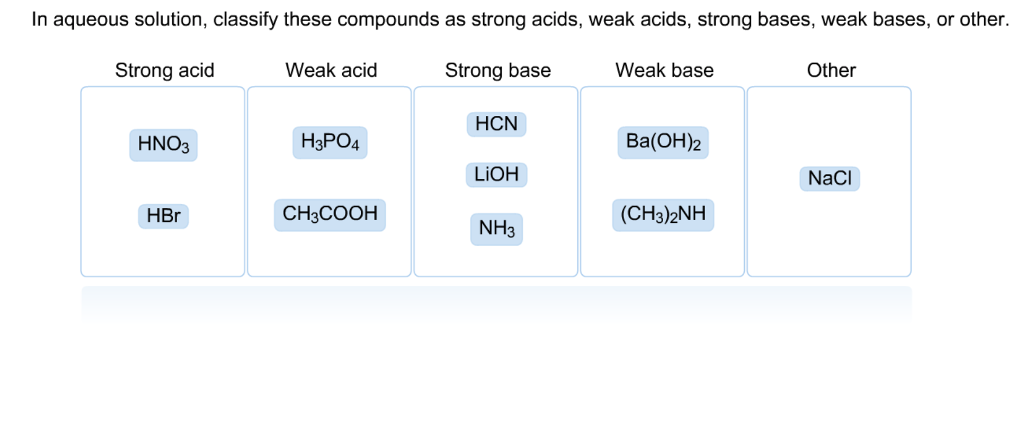
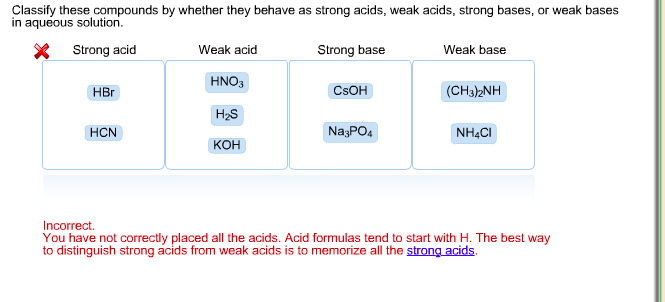
SOLVED: Classify these compounds by whether they behave as strong acids, weak acids, strong bases, Or weak bases in aqueous solution. Strong acid Weak acid Strong base Weak base KOH HNOz HzCOz

Which should be stronger acid, HOCN, or HCN? Explain briefly In HOCN, the H+ ion is attached to th - YouTube