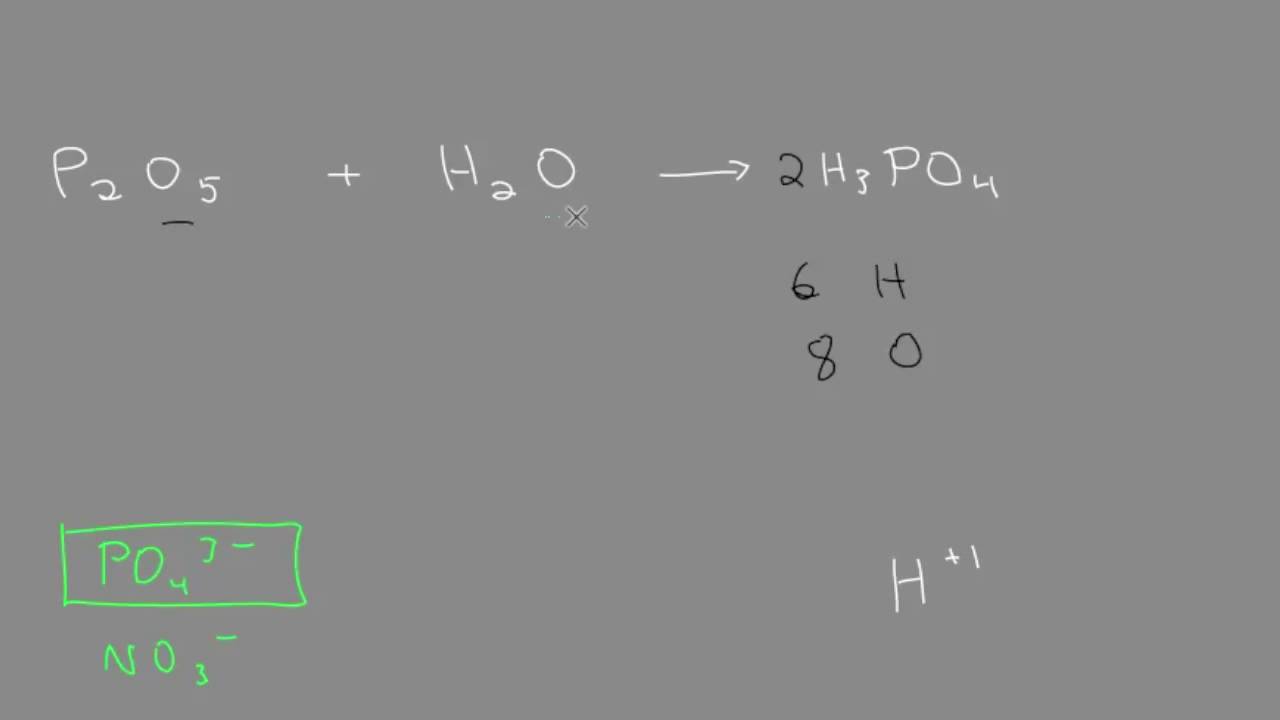From what I know...acid +base=salt +water..but the option confused me...the PH should be 7 right? and it must be metal oxide right? : r/igcse

Guys/Maam pls help me with this IN the given picture, the statement has given that Metal oxides - Chemistry - - 15057951 | Meritnation.com

Difference Between Metal Oxides and Non Metal Oxides | Definition, Properties, Different Types, Differences

Water Oxidation Catalysis: Electrocatalytic Response to Metal Stoichiometry in Amorphous Metal Oxide Films Containing Iron, Cobalt, and Nickel | Journal of the American Chemical Society

Metal sorption studies biased by filtration of insoluble metal oxides and hydroxides - ScienceDirect
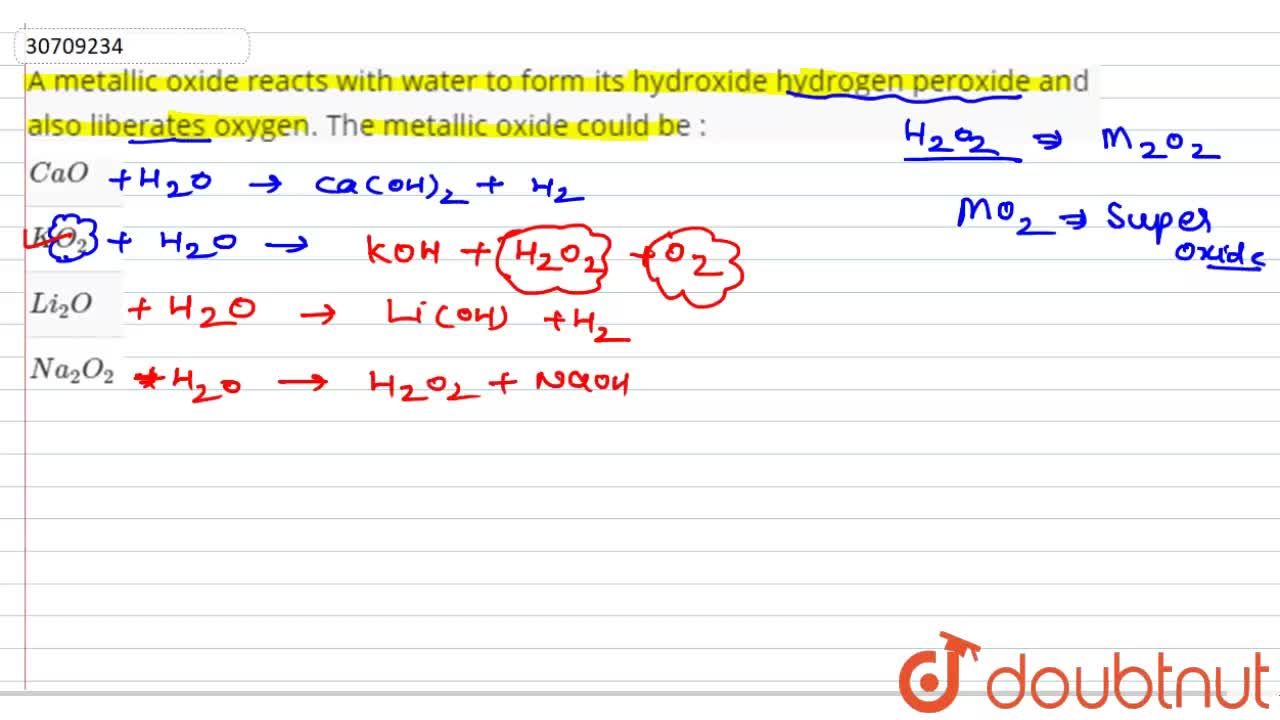
A metallic oxide reacts with water to form its hydroxide hydrogen peroxide and also liberates oxygen. The metallic oxide could be :
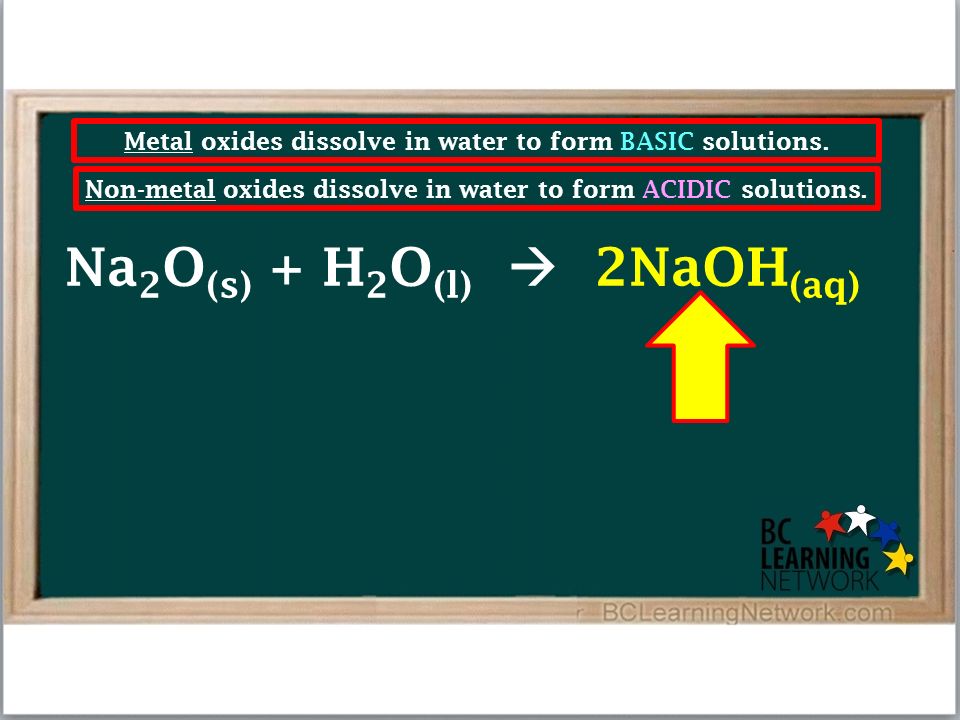
Metal and Non-metal Oxides. An oxide is a compound of oxygen and one or more other elements. - ppt download
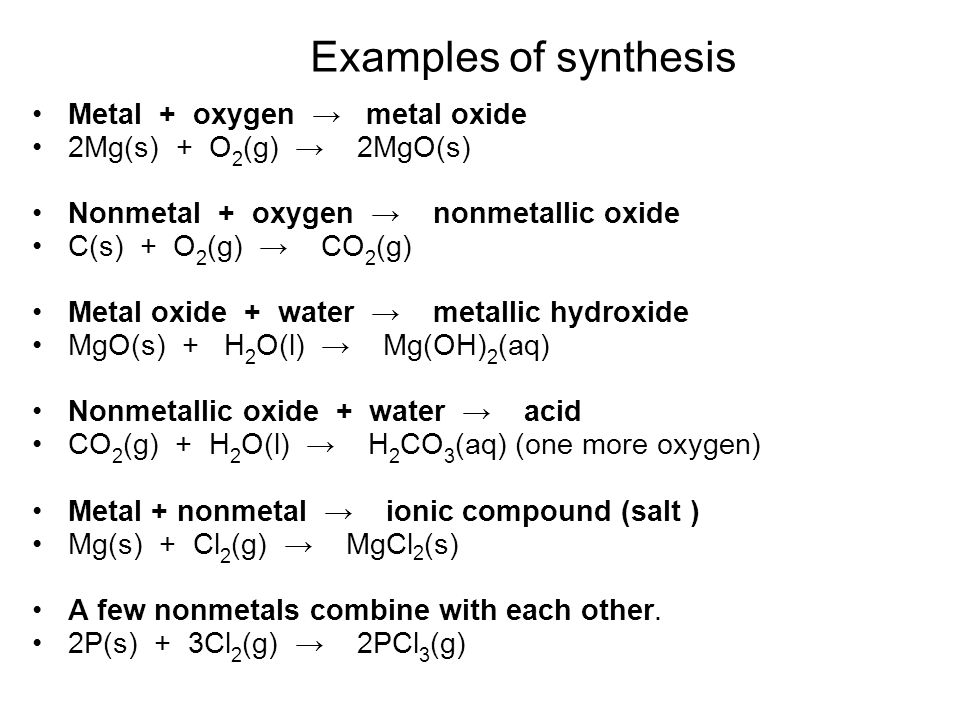
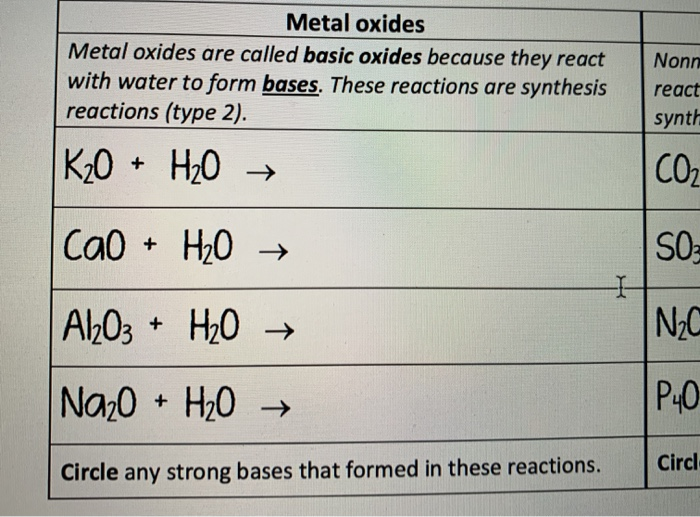
Types of Chemical Reactions. Synthesis Reactions Smaller atoms/compounds combine to form larger molecules Also known as combination or addition reactions. - ppt download

A metallic oxide reacts with water to form its hydroxide, hydrogen peroxide and also liberates oxygen. The metallic oxide could be :
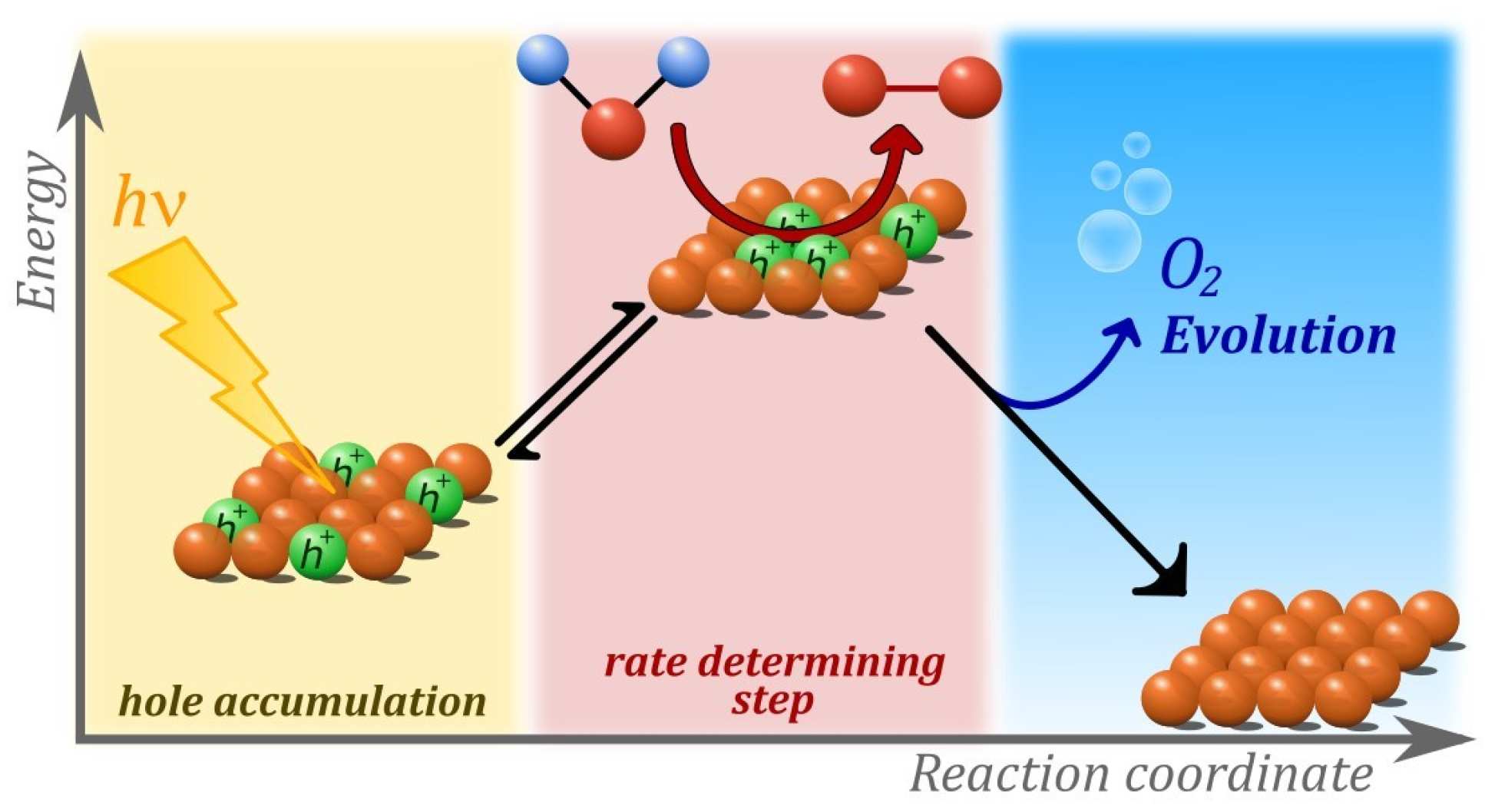
It takes three to make oxygen from water on metal oxides under sunlight | Imperial News | Imperial College London

5.2 - Salts Salts are a class of ionic compounds formed when: acids and bases react oxides or carbonates react with acids metals react with acids. - ppt download

![MCQ] Which of the statements is not correct? All metal oxides react MCQ] Which of the statements is not correct? All metal oxides react](https://d1avenlh0i1xmr.cloudfront.net/0146fde4-9748-4104-875a-ccbefb146ee1/reaction-of-metal-carbonate-with-acid---teachoo-01.jpg)



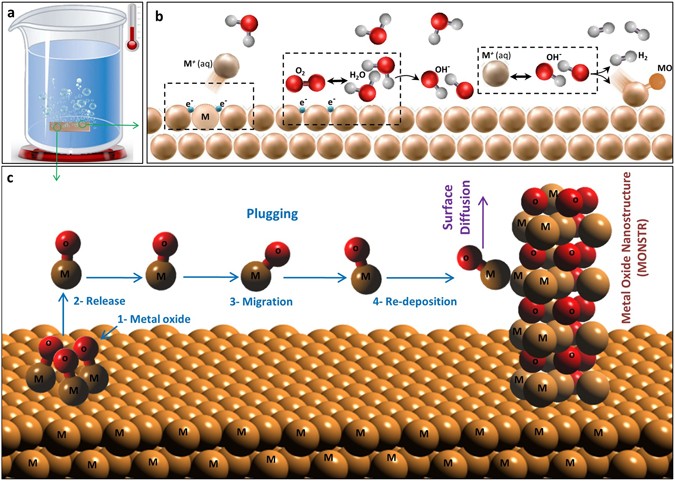

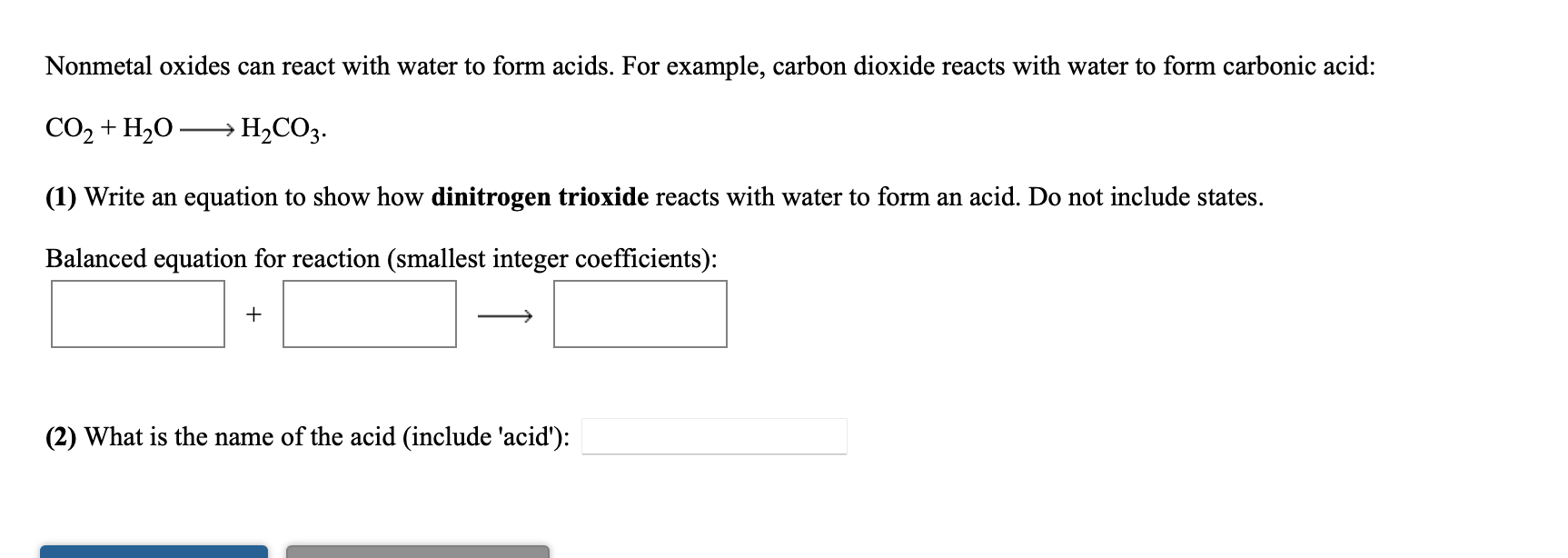



![MCQ] Which of the statements is not correct? All metal oxides react MCQ] Which of the statements is not correct? All metal oxides react](https://d1avenlh0i1xmr.cloudfront.net/1942b2cf-3ca7-4b5e-9607-79be588ba41c/reaction-of-non-metals-oxides-with-water---teachoo.jpg)